S. Nagy,1 Q. Xuebin,2 H. Jianlin2 and A. Kovács3
1. Institute of Animal Breeding, Faculty of Agricultural Sciences, University of West Hungary, H-9200 Mosonmagyaróvár, Vár 4., Hungary
2. Department of Animal Science, Gansu Agricultural University, Lanzhou 730070, Gansu, P.R. China
3. Department of Cell Biology, Institute for Animal Breeding and Nutrition, H-2053 Herceghalom, Gesztenyés út 1-3, Hungary
A simple, light microscopic sperm staining method was used for simultaneous evaluation of acrosome integrity, viability and morphology of sperm cells of wild and wild × domestic F1 yak bulls.
Keywords: Light microscopy, sperm quality, yak
A simple sperm staining method is used routinely for simultaneous evaluations of acrosome integrity, viability and morphology in Hungary (Kovács and Foote 1992). The aim of this pilot study was to try this method for frozen-thawed yak semen and to get some basic information for a wide-scale investigation on wild and domestic yak semen quality.
Viability testing stain: 0.20% trypan blue (prepared from 0.4% trypan blue, Sigma T 8154 diluted 1:1 with 0.9% NaCl). This solution is stable for several months at room temperature.
The fixative is composed of 86 mL of 1 N HCl plus 14 mL 37% formaldehyde solution and 0.2 g neutral red (Sigma N 2880). It is stable for about two months at room temperature and may be used repeatedly.
The acrosome stain is 7.5% Giemsa stock solution (Sigma GS 500) in distilled water prepared freshly before use.
Ten samples of frozen yak sperm (three wild and seven wild × domestic F1 yak) were thawed and diluted 10× with 0.9% NaCl at 38°C (two pellets to two mL NaCl-solution).
Equal drops of 0.2% trypan blue, and diluted semen were mixed on slides with the edge of another slide and smeared. They were air-dried in a near-vertical position at room temperature. They were fixed for 2 minutes. Slides were rinsed with tap and distilled water, and stained in Giemsa solution at room temperature overnight. They were rinsed with tap and distilled water, differentiated in distilled water for 2 minutes, and air-dried.
Following mounting with DPX (BDH, 360294H) and coverslipping, stained smears were evaluated by a light microscope at 400× magnification using a 40× objective. Three hundred spermatozoa per slide were counted.
For live/dead assessment, the posterior, for the status of the acrosome, the anterior part of the sperm head provides information (Kovács and Foote 1992).
Morphologically abnormal sperm cells were classified according to Barth and Oko (1989).
With this staining method, all sperm cell types described previously for other domestic animal species were clearly distinguished (Figure 1), including stained-membrane damaged-sperm tails (Nagy et al. 1999).
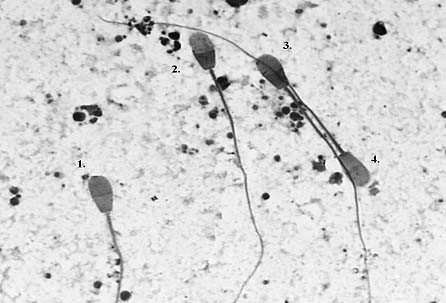
1. ‘Live’ cell, intact acrosome, unstained tail.
2. ‘Dead’ cell, lost acrosome.
3. ‘Dead’ cell, loose acrosome.
4. ‘Live’ cell, damaged acrosome, stained tail.
Figure 1. Yak sperm cells, Kovács–Foote staining.
Du (1987, cited by Cai and Wiener 1995) and Lu and Zhang (1994) mentioned the low rate of morphological abnormalities in the wild yak semen. In this study, the average rate of sperm head and tail defects and cytoplasmic droplets were 1.3%, 2.6% and 0.1%, respectively. The rate of total sperm defects was 4.0% (2.0–8.3%).
Percentage of 'live' (intact head, tail, and acrosome membrane) spermatozoa without morphological abnormalities can be a practical index of semen quality.
On the basis of the results and experiences of this pilot study, a larger project could be planned to make comparisons of wild and domestic yak semen samples, and to investigate the effect of the freezing method on the quality of the semen.
The authors are grateful to Prof Lu Zhonglin for kindly supplying the frozen semen for this study.
Barth A.D. and Oko R.J. 1989. Abnormal morphology of bovine spermatozoa. Iowa State University Press, Ames, IA, USA. 285 pp.
Cai L. and Wiener G. 1995. The yak. FAO (Food and Agricultural Organization of the United Nations) Regional Office for Asia and the Pacific, Bangkok, Thailand. 237 pp.
Kovács A. and Foote R.H. 1992. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotechnic and Histochemistry 67:119–124.
Lu Z.L. and Zhang J.W. 1994. Analysis of high reproductivity on wild yak bull. In: Rongchang Zh., Jianlin H. and Jianping W. (eds), Proceedings of the 1st international congress on yak held in Lanzhou, P.R. China, 4-9 September 1994. Supplement of Journal of Gansu Agricultural University, Lanzhou, P.R. China. pp. 287–290.
Nagy Sz., Házas G., Bali Papp Á., Iváncsics J., Szász F., Szász F. Jr. , Kovács A. and Foote R.H. 1999. Evaluation of sperm tail membrane integrity by light microscopy. Theriogenology 52:1153–1159.