R. Tamás,1 Q. Xuebin,2 P.T. Erika,3 N. Szabolcs,4 H. Jianlin,2 K. András,5 T. András3 and S. András1
1. Department of Biochemistry and Food Technology, Budapest University of Technology and Economics, H-1111 Budapest Muegyetem rkp. 3., Hungary
2. Department of Animal Science, Gansu Agricultural University, Lanzhou 730070, Gansu, P.R. China
3. Department of Obstetrics and Gynaecology, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
4. Department of Animal Genetics, Institute of Animal Husbandry, University of West Hungary, Mosonmagyaróvár, Hungary
5. Research Institute for Animal Breeding and Nutrition, Herceghalom, Hungary
Y-bearing bovine spermatozoa may be distinguished by fluorescence in situ hybridisation (FISH) using specific DNA probe, offering an in vitro control of experiments for producing sex-oriented or sex-specific semen. Bovine Y-specific probe gave distinguishable signal also in yak spermatozoa. Specific binding of the probe to the corresponding sex chromosome was confirmed on yak chromosome preparations.
Keywords: Cattle, fluorescence in situ hybridisation, sexing, spermatozoa, yak
Cattle (Bos taurus, L.) and yak (B./Poephagus grunniens, L.) are two related species having identical diploid chromosome number (2n = 60; Krallinger 1931; Zuitin 1935). The autosomes are all acrocentric, the X-chromosome is a large sub-metacentric in both species, the Y-chromosome of yak is small sub-metacentric (Popescu 1969; Kovács et al. 1982) demonstrating some polymorphism (Gang et al. 1991 cited by Jianlin 1996), similar to European cattle (B. taurus typicus).
Y-bearing cattle spermatozoa may be identified by fluorescence in situ hybridisation (Hassanane et al. 1998; Tardy et al. 1998; Hassanane et al 1999; Révay et al. 2000). Based on the morphologically similar sex-chromosomes, we tried to apply bovine Y-specific probes for sexing yak spermatozoa. Our goal was to elaborate a rapid and effective in vitro technique to control the experiments on producing sex-oriented yak semen (Cao et al. 1996).
Frozen/thawed yak semen doses were diluted with 0.9% NaCl, smeared, air-dried in China and mailed to Hungary. To facilitate the penetration of the probe and better signal visualisation, sperm heads were decondensed with papain-dithiothreitol solution (Schwerin et al. 1991; Hassanane et al. 1999).
Chromosome preparations were made from blood taken from a yak bull in Hungary (Moorhead et al. 1960).
The Y-chromosome specific probe was prepared by polymerase chain reaction (PCR) method. The primers are specific to the BC 1.2 region on the bovine Y-chromosome (Cotinot et al. 1991; Schwerin et al. 1991). The reaction was carried out using bovine male genomic DNA as template. The sequences of the oligonucleotide primers were: 5'ATC AGT GCA GGG ACC GAG ATG 3' and 5'AAG CAG CCG ATA AAC ACT CCT T 3'. The PCR was performed as described by Kobayashi et al. (1998) with modified MgCl2 concentration (1.2 mM). The 250 bp product was labelled with biotin-16-dUTP during the PCR.
FISH was performed as described by Pinkel et al. (1986) with slight modifications. The biotin labelled probe was detected with the Tyradmide Signal Amplification System (Direct FITC, NEN Life Sciences) according to the manufacturer. The slides were examined under a fluorescent microscope (Zeiss Opton) with simple microscopic observation.
Decondensation of yak spermatozoa needed a prolonged treatment as compared to cattle; 15–25 vs. 3–6 minutes. The bovine Y-probe produced strong signals in decondensed yak spermatozoa (Figure 1). Specificity of the probe was confirmed on yak metaphase chromosomes: the Y-chromosome was labelled with the probe, and there was no signal on the autosomes (Figure 2).
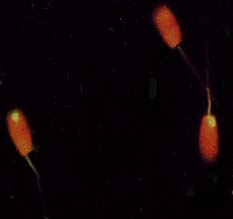
Figure 1. Detection of the bovine Y-probe on yak spermatozoa.
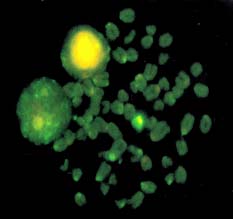
Figure 2. Detection of the bovine Y-probe on yak metaphase chomosome preparation.
We can conclude that it is possible to use the bovine Y-probe to control experiments on producing sex-oriented yak semen. Further effort will be needed to prepare bovine X-chromosome specific DNA probe, which is easy to produce and allow simultaneous detection of both the sex chromosomes on yak spermatozoa.
We would like to express our sincere thanks to Prof Lu Zhonglin for supplying yak semen for this study. This work was supported by the Hungarian Academy of Sciences (OTKA grant T 025300). We thank Prof Dohy János academician, head of the Agricultural Committee of the Hungarian Academy of Sciences for supporting our work from his grant from the Hungarian Ministry of Education (FKFP 0468/1997).
Cao Shizhen, Chen Liang, Han Jianlin, Ma Haimin and Wang Zhiwen. 1996. Preliminary separation of yak X- and Y-chromosomal bearing sperm by centrifugation on discontinous Percoll density gradients. International Yak Newsletter 2:44–46.
Cotinot C., Kirszenbaum M., Leonard M., Gianquinto L. and Vaiman M. 1991. Isolation of bovine Y-derived sequence: Potential use in embryo sexing. Genomics 10:646–653.
Hassanane M., Kovács A., Laurent P., Lindblad K. and Gustavsson I. 1998. Simultaneous detection of X- and Y-bearing bull spermatozoa by double colour fluorescence in situ hybridization. 13th European colloquium on cytogenetics of domestic animals, Budapest, 02-05 June, 1998. Hungarian Journal of Animal Production 48(1):128.
Hassanane M., Kovács A., Laurent P., Lindblad K. and Gustavsson I. 1999. Simultaneous detection of X- and Y-bearing bull spermatozoa by double colour fluorescence in situ hybridization. Molecular Reproduction and Development 53:407–412.
Jianlin H. 1996. Yak genetic resources in China: evaluation of chromosome, protein and mtDNA polymorphism. In: Miller D.G., Craig S.R. and Rana G.M. (eds), Proceedings of a workshop on conservation and management of yak genetic diversity held at ICIMOD, Kathmandu, Nepal, 29-31 October 1996. ICIMOD (International Centre for Integrated Mountain Development), Kathmandu, Nepal. pp. 175–183.
Kobayashi J., Sekimota A., Uchida H., Wada T., Sasaki K., Umezu M. and Sato E. 1998. Rapid detection of male-specific DNA sequence in bovine embryos using fluorescent in situ hybridization. Molecular Reproduction and Development 51:390–394.
Kovács A., Graf Z., Balázs K. and Fischer A. 1982. Chromosomal investigation of mammals in Budapest Zoo. Verhandlungsbericht des XXIV. Internationalen Symposiums über die Erkrankungen der Zootiere, Veszprém. Hungary 1982. Akademie-Verlag Berlin, GDR. pp. 475–482.
Krallinger H.F. 1931. Cytologische Studien an einigen Haussäugetieren. Archiv Tierernährung, Tierzuchtung Abteilung Band 5:127–187.
Moorhead P.S., Novell P.C., Mellmann W.J., Battips D.M. and Hungerford D.A. 1960. Chromosome preparation of leukocytes cultured from human peripheral blood. Experimental Cell Research 20:613–616.
Pinkel D., Straume T. and Gray J.W. 1986. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proceedings of National Academy of Sciences (USA) 83:2934–2938.
Popescu C.P. 1969. Idiograms of yak, cattle and their hybrids. Annales de Génétique et de Sélection Animale 1:207–217.
Révay T., Tardy E.P., Tóth A., Kovács A. and Salgó A. 2000. Sexing bovine cells by FISH with a synthetic Y-probe. 14th European colloquium on cytogenetics of domestic animals. Brno, Czech. 27–30 June, 2000.
Schwerin M., Blottner S., Thomsen P.D., Roschlau D. and Brockmann G. 1991. Quantification of Y chromosome bearing spermatozoa of cattle using in situ hybridization. Molecular Reproduction and Development 30:39–43.
Tardy E.P., Szalai G., Gustavsson I., Hassanane M., Lindblad K., Kovács A., Házas G., Tóth A. and Dohy J. 1998. First results of sexing bovine spermatozoa by FISH in Hungary. 13th European colloquium on cytogenetics of domestic animals, Budapest, Hungary, 02-05 June 1998. Hungarian Journal of Animal Production 48(1):128–129.
Zuitin A.I. 1935. On the chromosomes of yak (Poephagus grunniens, L.). C.R. (Dokl.) Acad. Sci. U.S.S.R., N.S. 4, 81–83. In: Hulot F. and Lauvergne J.J. Les chromosomes des ruminants. Annales de Génétique 10:86–97.